Brandenburg Kapital - News
SphingoTec launches novel biomarker test for intensive care patients on diagnostic point-of care platform
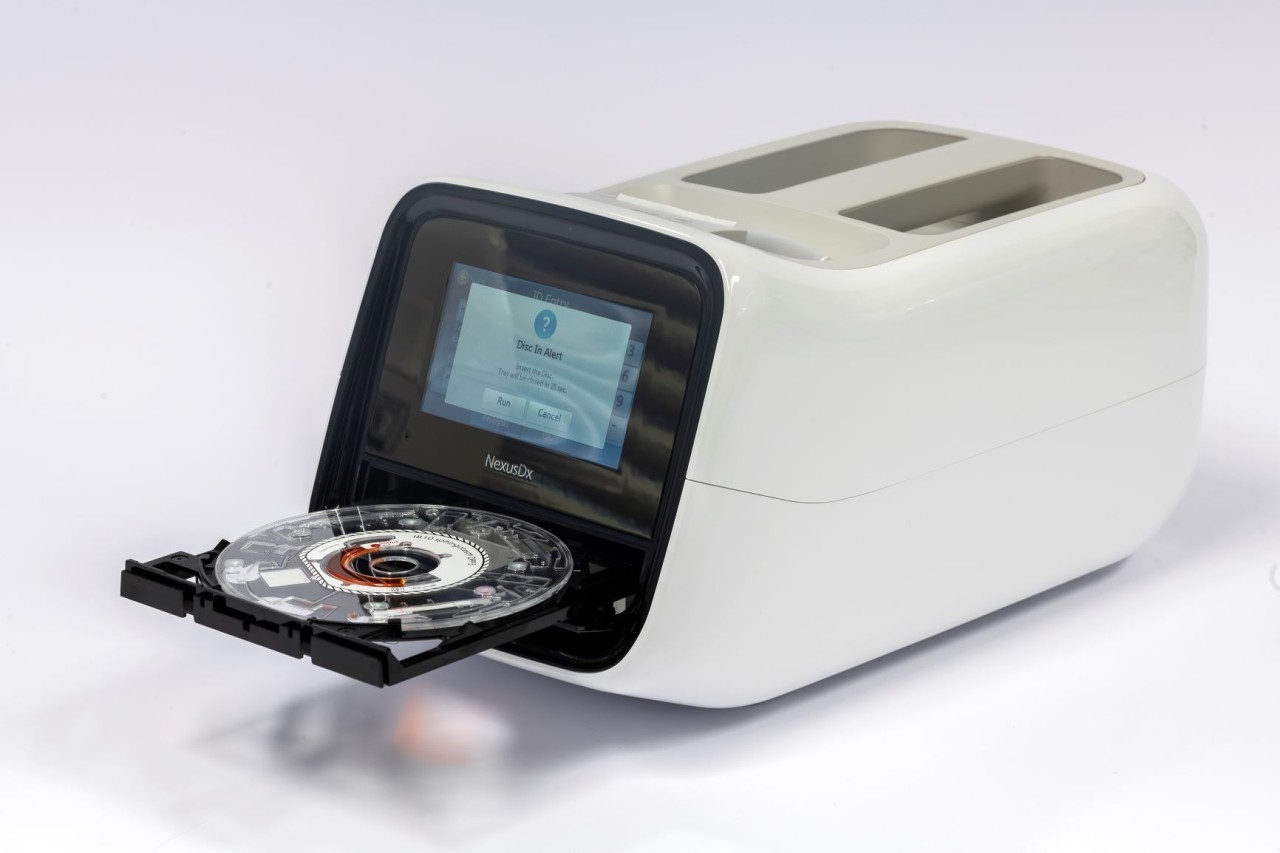
Potsdam – September 2019 Diagnostics company SphingoTec GmbH (SphingoTec), Hennigsdorf near Berlin, launched their novel and proprietary biomarker test IB 10 sphingotest® DPP3. The test is applicable patient-side at Nexus IB 10 point-of-care platform. IB 10 sphingotest® DPP3 is the first CE-marked point-of-care biomarker test able to quantify DPP3 blood-plasma levels. DPP3 is a novel and unique biomarker predicting the course of diseases in patients with acute medical conditions as for example cardiogenic shock already at admission to the emergency room or intensive care unit.
About SphingoTec GmbH
Founded in 2002 SphingoTec develops and markets innovative in vitro diagnostic IVD tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as acute heart failure acute kidney injury as well as septic shock in order to support patient management and provide guidance for treatment strategies. Along with point-of-care-Plattform Nexus IB 10 by its wholly owned subsidiary Nexus Dx Inc., an instant and patient-side diagnosis and analysis can be conducted. In addition and in order to support prevention strategies, SphingoTec developed a portfolio of novel biomarkers, which predict the risks of obesity, breast cancer and cardiovascular diseases.
Find more information at www.brandenburg-kapital.de and www.sphingotec.com